美国“疟疾”在研基金,高达272项!这个药物遗传学、耐药表观遗传学的课题设计,值得所有医生借鉴(2024)
时间:2024-09-30 18:00:58 热度:37.1℃ 作者:网络
疟疾(Malaria)是一种由寄生虫(疟原虫)引起的传染病,主要通过感染了疟原虫的雌性按蚊叮咬传播。这种疾病在全球范围内尤其影响热带和亚热带地区,对公共卫生构成重大挑战。疟疾的典型症状包括发热、寒战、头痛、恶心和呕吐,症状周期性出现,特别是每次发热周期后病人可能感到极度疲劳。
尽管近年来在疟疾的诊断和治疗方面取得了显著进展,但仍存在一些重要而未解决的临床问题:
-
药物耐药性:疟疾治疗面临的最大挑战之一是疟原虫对现有抗疟药物的耐药性不断增强,尤其是对氯喹和阿莫地喹。这一问题严重阻碍了疟疾控制和根除努力。
-
有效疫苗的开发:尽管有疟疾疫苗(如RTS,S/AS01疫苗)的研发和部分应用,但现有疫苗的保护效果有限且持续时间不长。开发一个高效和长效的疟疾疫苗是一个紧迫的全球健康需求。
-
病媒控制:有效控制疟疾的传播需要有效的病媒(按蚊)控制策略。然而,抗药性的增加和适合所有地区的控制策略的缺乏使得病媒控制更加困难。
-
快速诊断:在资源有限的地区,快速准确地诊断疟疾仍然是一个挑战。提高快速诊断测试的准确性和可及性对于及时治疗和控制疟疾传播至关重要。
解决这些问题需要国际合作、科技创新和公共卫生策略的持续改进。全球健康组织和研究机构需要共同努力,以发展新的治疗方法、提高疫苗的有效性、优化病媒控制技术,并改进疟疾的诊断工具。
我们仅对美国国立卫生研究院(NIH)资助的在研疟疾相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Malaria”为检索词、在题目中进行检索,美国NIH针对疟疾的在研有272项。
一,谁获得了这些研究?
1,在研疟疾基金最多的PI
-
NIAID EXTRAMURAL ACTIVITIES 的 DUFFY, PATRICK
-
SANARIA, INC. 的 HOFFMAN, STEPHEN LEV
-
NIAID EXTRAMURAL ACTIVITIES 的 PIERCE, SUSAN
-
NIAID EXTRAMURAL ACTIVITIES 的 CROMPTON, PETER
-
SEATTLE CHILDREN'S HOSPITAL 的 STUART, KENNETH D
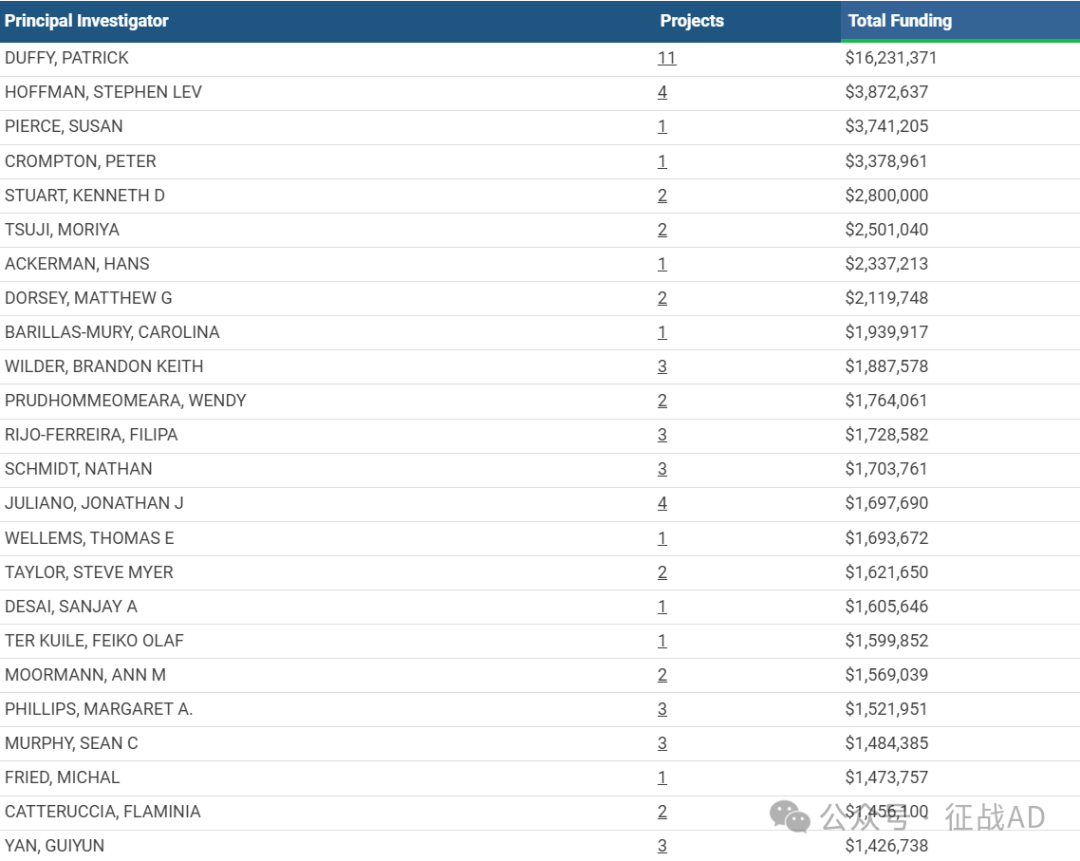
2,疟疾基金最多的研究机构
-
美国国家过敏和传染病研究所
-
加州大学旧金山分校
-
哥伦比亚大学健康科学系
-
杜克大学
-
西雅图儿童医院等
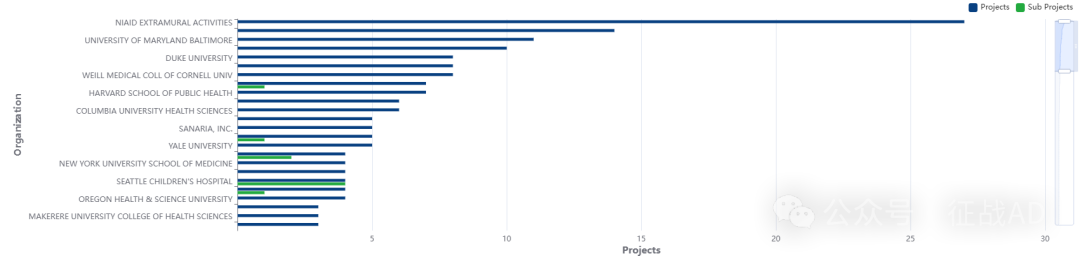
二,疟疾研究热点是什么?
疟疾研究领域总览(根据关键词)
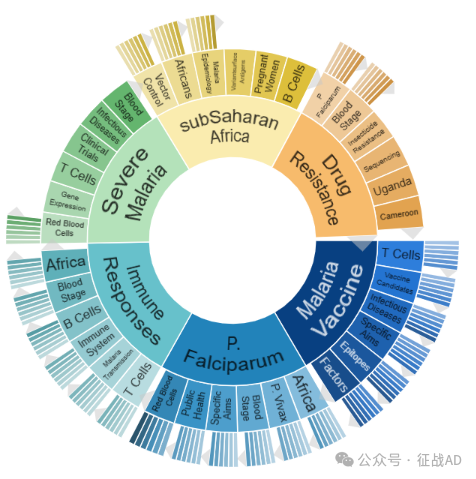
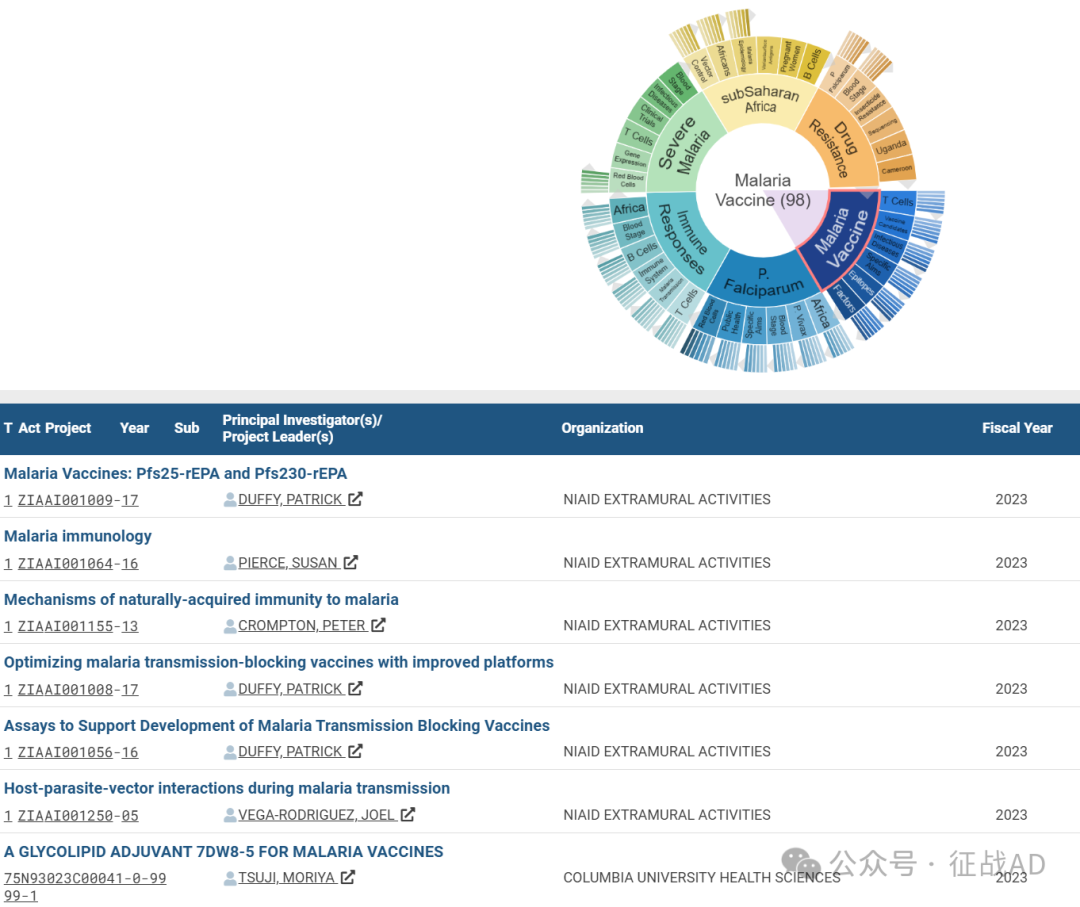
A,关于疟疾疫苗(Malaria Vaccine)的研究项目最多
有 98 项在研基金涉及到了疟疾疫苗,关注最多的方面包括T细胞(T Cells)、疫苗候选物(Vaccine Candidates)、传染病(Infectious Diseases)、具体目标(Specific Aims)、表位(Epitopes)、因素(Factors)等方面研究。
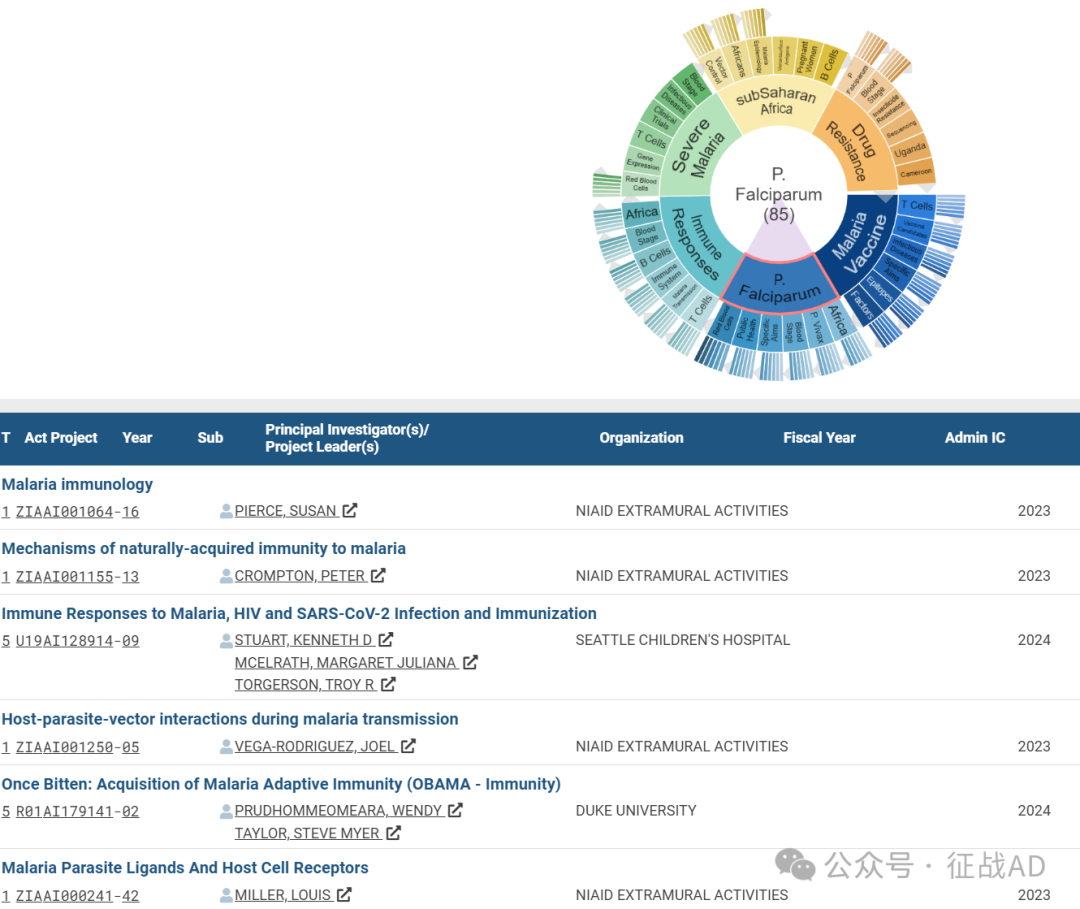
B,恶性疟原虫(P. Falciparum)的研究
有 85 项研究涉及到恶性疟原虫,研究领域主要涉及非洲(Africa)、间日疟原虫(P. Vivax)、血液阶段(Blood Stage)、具体目标(Specific Aims)、公共卫生(Public Health)、红细胞(Red Blood Cells)等方面研究。
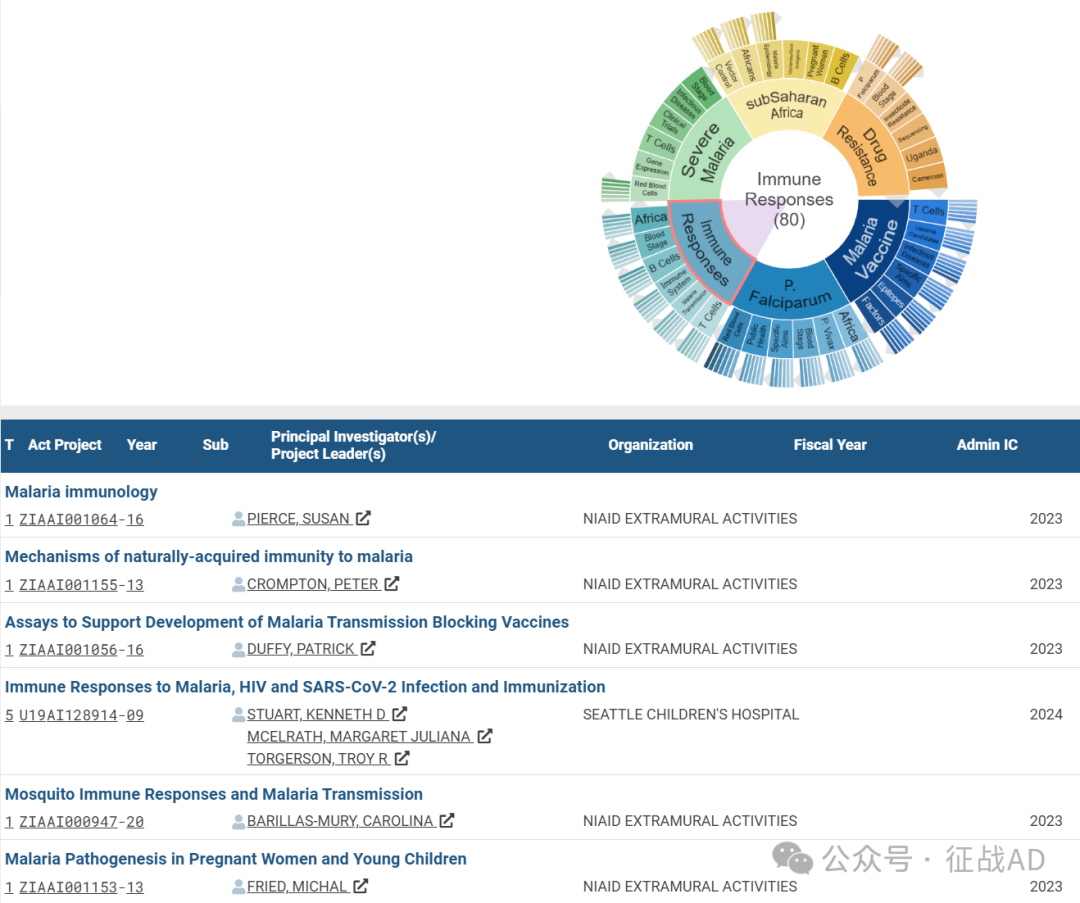
C,免疫反应(Immune Responses)
有 80 项研究涉及到免疫反应,涉及的关键词包括如T细胞(T Cells)、疟疾传播(Malaria Transmission)、免疫系统(Immune System)、B细胞(B Cells)、非洲(Africa)、血液阶段(Blood Stage)等。
其他疟疾研究大的方向也包括重症疟疾(Severe Malaria)、撒哈拉以南非洲(SubSaharan Africa)、耐药性(Drug Resistance)等。
三,借鉴与突破
我们也分享在疟疾领域的几项课题摘要,希望对同仁们有所启发。
A,Harnessing the Power of Experimental Genetic Crosses to Probe Drug Resistance in Malaria
In the initial funding period of this Program Project (P01), we made great strides in transitioning Plasmodium crosses from the original model using chimpanzees to the human-liver chimeric mouse infused with human red blood cells (the FRG huHep/huRBC mouse) to generate more than 30 crosses in a span of 5 years. This included many replicate crosses of 6 different parental combinations that produced nearly a thousand new cloned progeny. This capacity allowed for optimizations that drove our development of the bulk segregant analysis (BSA) methodology and put within reach the ability to use experimental crosses of new clinical isolates to test specific hypotheses about emerging drug resistance. The combination of routine and replicated experimental crosses with BSA has shifted the challenge from making crosses and identifying genetic loci to instead prioritizing the rapid identification of genes and mechanisms underling parasite drug resistance and fitness. Our capstone discovery identified the role of pfaat1 as an epistatic partner with pfcrt in the evolution of chloroquine resistance that influences the balance between resistance and compensatory fitness, a crucial determinant of how new drug resistances emerge and spread.
The overall goal of this P01 renewal proposal is to continue to advance this technology to track in real-time the alarming emergence of artemisinin resistance (ART-R) and its partner drugs used in artemisinin combination therapies (ACTs) in East Africa, an imminent threat to reverse decades of progress against morbidity and mortality due to malaria on the continent where more than 90% of malaria deaths occur. Coding mutations in the kelch13 gene that are strongly associated with ART-R and serve as the only markers for surveillance. However, mutations in kelch13 generate a wide range of resistance levels and fitness effects, and how these effects are compensated by other structural or regulatory changes in the genome remains unknown.
Relying on 3 Research Projects, supported by 2 Scientific Cores, we will use targeted experimental genetic crosses to (i) dissect the genetic complexity of ART-R, (ii) clarify the role of kelch13, (iii) define the regulators and partner genes that control ART-R and emerging resistance to lumefantrine, the predominant ACT partner drug in Africa and (iv) establish a sustainable and user-centric valuable community resource including our methodological pipeline, data and biological materials. In the process, we will expand our BSA drug selection protocols to include cutting edge single cell RNAseq as the next step in building this powerful community resource for real-time solutions to clinically urgent questions.
B, Epigenetic drivers of quiescence in artemisinin-resistant Plasmodium falciparum malaria
We recently uncovered one such mechanism, via tRNA modification reprogramming, that drug-resistant parasites use to rapidly alter their proteome in a codon- bias manner as a ‘just-in-time’ translational stress response. We hypothesize that, similar to quiescent tumor cells and bacterial persister cells, Pf can also use noncoding RNAs (ncRNAs) to alter its transcription and translation in response to stress. Uncovering these yet unidentified mechanisms will fundamentally alter how we understand transcriptional and translational control in malaria parasites.
By harnessing techniques from tumor biology and bacterial persister systems, we propose to identify and functionally characterize the roles of ncRNAs in parasite quiescence. We will use transcriptomics to identify differentially changing long ncRNAs and tRNA- derived fragments in ART-resistant and ART-sensitive parasites after exposure to ART or to isoleucine starvation. Candidate ncRNAs will be validated using complementary approaches, including CRISPR/Cas9 conditional knockdowns and in vitro parasite drug susceptibility assays. Molecular mechanisms will be elucidated using biotinylated candidate ncRNAs as bait to identify interacting RNA binding proteins by mass spectrometry (MS). We will also test the hypothesis that modifications on the amino-acyl tRNA can regulate ART-mediated quiescence at the translational level by using a combination of specialized MS and genetic knockdown approaches. Finally, we will create a novel quiescent reporter parasite line, allowing for live cell sorting of quiescent parasites. This will provide the first opportunity to examine transcriptomic changes at the single cell level as parasites enter and exit quiescence.
We anticipate that our findings will shift the paradigm of how we understand gene regulation in drug-resistant, blood stage Pf and that the framework and tools generated herein can be expanded to other quiescent stages of the parasite lifecycle. Our results will also identify parasite vulnerabilities that can be leveraged into new strategies to treat ART-resistant malaria.


